-

-
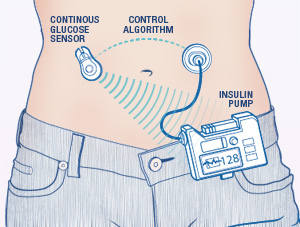
The AP SmartPhone is secure inside a prototype box, affectionately nicknamed ” THE BRICK ” by the UVA research team. Brick 1 also contains my CGM and Bluetooth communications back to the trial control computers.
-
-
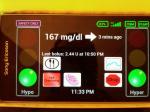
AP SmartPhone shows Flat Line 167 mg/dl of awesome glucose control after dinner and prior to bedtime on March 14, 2013.
-
-
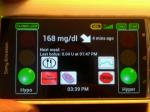
The AP Smartphone shows the obvious benefits of my 45 minute walk in Charlottesville’s Downtown Mall. AP is adjusting background insulin dose to bring my post-lunch glucose down and keep me from a crashing low.
-

-
Boris P. Kovatchev, Ph.D, and I show off the AP after my afternoon walk. Dr. Boris is the Director of the UVA Center for Diabetes Technology and the person who is building the ” brain ” or algorithm that makes the AP possible.
-

-
Dr. Daniel Chernavvsky, Clinical Research Coordinator, and I display the AP as the 24-hour clinical trial ends March 15. I did NOT want to give it back to him. I’m sure he was watching me closely, LOL.
-

-
Before leaving UVa and giving back the AP, I pose with a few of the team members who watched over me and conducted the outpatient trial. L to R: my nurse, Crystal Leathers, computer engineer Benton MIze, and Stacey Anderson, MD.
OK, so the headline might be a little bit over the top. My apologies. However, I was wearing the ” Future ” of high tech diabetes self-management, and it was amazing, incredible and a breath of fresh air.
I was blessed to actually plug in and wear the Artificial Pancreas in a human clinical trial at UVa on March 14 and 15. The AP is not exactly like clicking the Arc Reactor into your chest, but the two devices are similar. It is a new, high-tech device that brings the promise of: better health; less daily stress for you and your family, and the powerful hope of fewer long-term medical complications from diabetes such as blindness, heart attacks, strokes and amputations.
For 24 wonderful hours, I felt like Iron Man — without the cool cars, the ability to fly, the Malibu mansion or the lifestyle of Robert Downey, Jr. But the experience was still “Super Hero-like” for someone like me who has battled the ” Super Villan” Type 1D for 14 years and been unable to achieve consistent glucose control. The AP is not a cure for diabetes, but it does offer a solution or pathway to better health outcomes for the 3 million Americans living with Type 1 diabetes.
After missing an opportunity to participate in the last AP trial, this time I passed the FDA’s strict health screening ( done May 11) and was invited by the research team at the UVA Center for Diabetes Technology to participate in a shortened outpatient trial. Recently, the UVA team successfully completed a 48-hour pilot study with 5 individuals — to prove that a ” wearable AP is feasible and safe; therefore its continued testing and refinement for outpatient use is warranted,” according to Boris P. Kovatchev, Ph.D., the lead investigator and Director of the center.
In the prior study, the AP participants led a ” normal ” life and were able to eat and sleep without the constant finger pricks and mental burden of ” how’s my sugar doing now?” Check out Tom Brobson’s video story of his UVa trial experience. This was the first out-patient study done in the United States.
As a follow-up to this clinical trial, my one-person study was not designed to test the AP algorithm or to determine if the software worked. We know the AP works — and it worked again for me in this trial! The UVa team called me for a ” dry run” test to see if a new hardware solution — a fix to provide an uninterrupted source of power to the AP and the CGM — would prevent the battery ” burn out” that happened during the previous trials. This is where ” BRICK 1 ” comes in. The researchers designed a new power and communication system to be combined within one unit. The good news: the ” Brick” designed appeared to work exactly as the researchers expected during my 24-hour test, providing consistent data reporting with no gaps!
The team put the AP SmartPhone, CGM, Bluetooth wireless communication equipment all together in ” Brick 1″ — this is what I carried with me and how I communicated with my insulin pump. And instead of staying put in a hospital or hotel room, I was able to carry the AP/CGM Brick with me as I walked to dinner at the College Inn on ” The Corner” across from campus and again for lunch on Friday. As Dr. Boris joked with the local NBC TV reporter, “this is the first time ever anyone with the artificial pancreas has been outside walking in a parking lot.” One small step for man, one giant leap for AP research!
Dr. Boris, Dr. Daniel and Benton Mize, the computer engineer working on the AP design, said the Federal Drug Administration (FDA) — which is regulating and monitoring all human clinical trials performed in Charlottesville and the 4 other AP consortium universities in the USA, France and Italy — needs clinical proof the technology works and that patients in non-hospital trials will be safe from low glucose episodes. The technology must give researchers uninterrupted remote monitoring capability of participants on a 24/7 basis when AP home trials start in 2014.
My bottom line — the AP worked as advertised. I was involved in determining my meal boluses and counting my carbs ( the control factor). The AP was able to operate in ” safe mode ” while I slept and ” closed loop” mode while I ate dinner or exercised. It essentially acted like a normal pancreas does providing sugar control automatically. The CGMs and the AP monitored my sugar every 5 minutes and adjusted by basal rates ( baseline insulin dose) up or down to help me achieve the best possible glucose control with the least amount of work.
And thanks to the AP and the awesome team at UVa, I had the freedom to go to bed and sleep all night with no 2 am lows. I maintained a Flat Line sugar reading overnight and woke up the same — and that’s a “Super Hero” result and the promise of a bright future for everyone living with Type 1 diabetes.
Next post: I’ll add a few more observations from my clinical trial; Dr. Daniel’s trip to the University of California for a new outpatient clinical trial with the AP and “BRICK 1”; and Dr. Boris’ talks with manufacturers to get better quality technology for the AP.